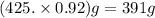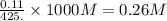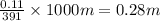A student dissolves 14.g of benzoic acid C7H6O2 in 425.mL of a solvent with a density of 0.92 g/mL. The student notices that the volume of the solvent does not change when the benzoic acid dissolves in it. Calculate the molarity and molality of the student's solution. Round both of your answers to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
A student dissolves 14.g of benzoic acid C7H6O2 in 425.mL of a solvent with a density of 0.92 g/mL....
Questions

Mathematics, 16.06.2020 18:57

English, 16.06.2020 18:57


Mathematics, 16.06.2020 18:57


English, 16.06.2020 18:57



Computers and Technology, 16.06.2020 18:57


English, 16.06.2020 18:57





Mathematics, 16.06.2020 18:57


Computers and Technology, 16.06.2020 18:57


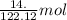 of benzoic acid = 0.11 mol of benzoic acid
of benzoic acid = 0.11 mol of benzoic acid