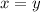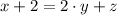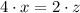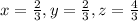Chemistry, 03.03.2020 03:33 dogsb4doods
Give the stoichiometric coefficient for oxygen when the following equation is balanced using the lowest, whole-number coefficients: ___CH4O(l) ___O2(g) -> ___CO2(g) H2O(l)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

You know the right answer?
Give the stoichiometric coefficient for oxygen when the following equation is balanced using the low...
Questions


History, 25.10.2019 06:43

History, 25.10.2019 06:43

History, 25.10.2019 06:43


Mathematics, 25.10.2019 06:43

Social Studies, 25.10.2019 06:43

Physics, 25.10.2019 06:43

English, 25.10.2019 06:43

Physics, 25.10.2019 06:43




Computers and Technology, 25.10.2019 06:43

Mathematics, 25.10.2019 06:43



Social Studies, 25.10.2019 06:43


Mathematics, 25.10.2019 06:43

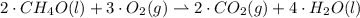
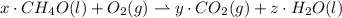
 are the required variables.
are the required variables.