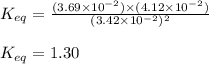Chemistry, 03.03.2020 03:22 icantspeakengles
Some CH2Cl2 is placed in a sealed flask and heated to 517 K. When equilibrium is reached, the flask is found to contain CH2Cl2 (3.42×10-2 M), CH4 (3.69×10-2 M), and CCl4 (4.12×10-2 M). What is the value of the equilibrium constant for this reaction at 517 K?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
Some CH2Cl2 is placed in a sealed flask and heated to 517 K. When equilibrium is reached, the flask...
Questions



Mathematics, 30.06.2019 20:30


Chemistry, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30




History, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30

Mathematics, 30.06.2019 20:30

Health, 30.06.2019 20:30


Mathematics, 30.06.2019 20:30


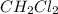 follows:
follows: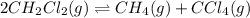
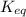 for above equation follows:
for above equation follows:![K_{eq}=\frac{[CH_4][CCl_4]}{[CH_2Cl_2]^2}](/tpl/images/0531/6381/cdd40.png)
![[CH_4]_{eq}=3.69\times 10^{-2}M](/tpl/images/0531/6381/50f3b.png)
![[CCl_4]_{eq}=4.12\times 10^{-2}M](/tpl/images/0531/6381/3e13d.png)
![[CH_2Cl_2]_{eq}=3.42\times 10^{-2}M](/tpl/images/0531/6381/c3f41.png)