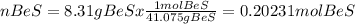Chemistry, 03.03.2020 02:47 averiemiranda1
The chemical formula for beryllium sulfide is BeS. A chemist measured the amount of beryllium sulfide produced during an experiment. She finds that 8.31 g of beryllium sulfide is produced. Calculate the number of moles of beryllium sulfide produced. Be sure your answer has the correct number of significant digits

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3


Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
The chemical formula for beryllium sulfide is BeS. A chemist measured the amount of beryllium sulfid...
Questions



Mathematics, 27.06.2019 08:00


Biology, 27.06.2019 08:00

Mathematics, 27.06.2019 08:00





Geography, 27.06.2019 08:00




Mathematics, 27.06.2019 08:00



Mathematics, 27.06.2019 08:00