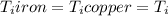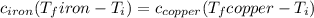Five-gram samples of copper and iron are at room temperature. both samples receive equal amounts of energy due to heat flow. the specific heat capacity of copper is 0.09 cal/g°c, and the specific heat capacity of iron is 0.11 cal/g°c. which of the following statements is true? the temperature of each sample will increase by the same amount. the temperature of each sample will decrease by the same amount. the copper will get hotter than the iron. the iron will get hotter than the copper.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Five-gram samples of copper and iron are at room temperature. both samples receive equal amounts of...
Questions

Chemistry, 29.06.2019 15:00

History, 29.06.2019 15:00

History, 29.06.2019 15:00

Mathematics, 29.06.2019 15:00


Chemistry, 29.06.2019 15:00



Chemistry, 29.06.2019 15:00


Social Studies, 29.06.2019 15:00



History, 29.06.2019 15:00


English, 29.06.2019 15:00

Mathematics, 29.06.2019 15:00

Mathematics, 29.06.2019 15:00

English, 29.06.2019 15:00

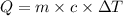
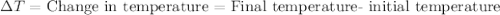
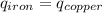
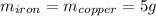 , also
, also