
Consider the following three processes: (1) Melting of ice at room temperature (2) Boiling of water at 101°C (3) Dissolving of NH4NO3 with water in an instant cold pack? Which statement(s) that are true about ALL three of the given processes? (Choose one or more)(A) Endothermic(B) Exothermic(C) Nonspontaneous(D) Spontaneous(E) none of these statements can be used to describe all three processes

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 13:30
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
Consider the following three processes: (1) Melting of ice at room temperature (2) Boiling of water...
Questions

Mathematics, 13.03.2021 01:00

Business, 13.03.2021 01:00



Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Biology, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00





Mathematics, 13.03.2021 01:00

Biology, 13.03.2021 01:00


History, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00



Biology, 13.03.2021 01:00

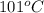 is also an endothermic process. This is because heat is absorbed by water molecules due to which their state changes from liquid to vapor form. When
is also an endothermic process. This is because heat is absorbed by water molecules due to which their state changes from liquid to vapor form. When 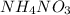 is dissolved in water then heat is absorbed as there occurs a decrease in temperature of water. Hence, it is also an endothermic reaction.
is dissolved in water then heat is absorbed as there occurs a decrease in temperature of water. Hence, it is also an endothermic reaction.

