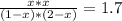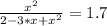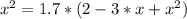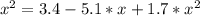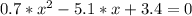Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2
You know the right answer?
The reversible chemical reaction A+B⇌C+D has the following equilibrium constant: Kc=[C][D][A][B]=1.7...
Questions



Mathematics, 27.04.2021 06:50



SAT, 27.04.2021 06:50



English, 27.04.2021 06:50

Mathematics, 27.04.2021 06:50

Mathematics, 27.04.2021 06:50

Mathematics, 27.04.2021 06:50

Mathematics, 27.04.2021 06:50


Social Studies, 27.04.2021 06:50


Mathematics, 27.04.2021 06:50

Mathematics, 27.04.2021 06:50


![Kc=\frac{[C]^{c} *[D]^{d} }{[A]^{a} *[B]^{b} }](/tpl/images/0531/2647/eda24.png)
![Kc=\frac{[C]*[D]}{[A]*[B]}=1.7](/tpl/images/0531/2647/e4842.png)