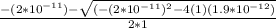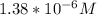Chemistry, 03.03.2020 01:01 ELGuapo6746
Calculate the pH at the equivalence point for the titration of 0.190 M 0.190 M methylamine ( CH 3 NH 2 ) (CH3NH2) with 0.190 M HCl . 0.190 M HCl. The K b Kb of methylamine is 5.0 × 10 − 4 .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identifying limitations of kinetic-molecular theorya chemist is studying the properties of a gas under various conditions. he observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. when the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal .
Answers: 1

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1


Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1
You know the right answer?
Calculate the pH at the equivalence point for the titration of 0.190 M 0.190 M methylamine ( CH 3 NH...
Questions


Mathematics, 25.01.2021 03:20

Chemistry, 25.01.2021 03:20

History, 25.01.2021 03:20




Mathematics, 25.01.2021 03:20


Mathematics, 25.01.2021 03:20

English, 25.01.2021 03:20

Health, 25.01.2021 03:20

History, 25.01.2021 03:20

Physics, 25.01.2021 03:30


Mathematics, 25.01.2021 03:30


Mathematics, 25.01.2021 03:30


Spanish, 25.01.2021 03:30

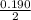
![K_a =\frac{[CH_3NH_2][H^+]}{[CH_3NH^+_3]}](/tpl/images/0531/3181/3871d.png)
![K_a = \frac{[x][x]}{[0.095-x]}](/tpl/images/0531/3181/0ca50.png)
![K_a = \frac{[x^2]}{[0.095-x]}](/tpl/images/0531/3181/85100.png) ------ equation (1)
------ equation (1)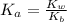
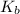 =
= 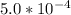 ; and
; and 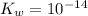 ;
;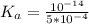
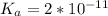
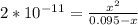
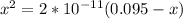
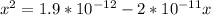

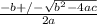 ; we have
; we have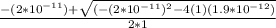 OR
OR