Chemistry, 02.03.2020 23:58 Dogtes9667
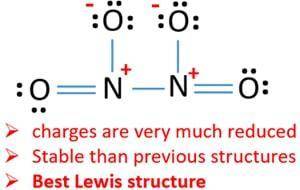
The N−N bond length in N2O is 1.12 Å, slightly longer than a typical N≡N bond, which is 1.10 Å, and the N−O bond length is 1.19 Å, slightly shorter than a typical N=O bond, which is 1.22 Å. Based on these data, which resonance structure best represents N2O?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
The N−N bond length in N2O is 1.12 Å, slightly longer than a typical N≡N bond, which is 1.10 Å, and...
Questions

Mathematics, 17.10.2020 15:01

History, 17.10.2020 15:01

Mathematics, 17.10.2020 15:01


Biology, 17.10.2020 15:01


Computers and Technology, 17.10.2020 15:01


History, 17.10.2020 15:01




Mathematics, 17.10.2020 15:01

English, 17.10.2020 15:01