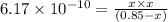Chemistry, 02.03.2020 23:29 ramirezzairap2u4lh
Use the "x is small" approximation to find the concentration of the products in the following reaction which initially contains only 0.85 M HCN. Decide whether using the approximation was valid or invalid. HCN(aq) ⇌ H+(aq) + CN−(aq), Kc= 6.17 x 10−10

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1


Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1
You know the right answer?
Use the "x is small" approximation to find the concentration of the products in the following reacti...
Questions

Mathematics, 08.01.2021 02:30

Mathematics, 08.01.2021 02:30

Mathematics, 08.01.2021 02:30



Chemistry, 08.01.2021 02:30




Mathematics, 08.01.2021 02:30



Mathematics, 08.01.2021 02:30




Mathematics, 08.01.2021 02:30



Mathematics, 08.01.2021 02:30

![[H^+]=0.0000229 M](/tpl/images/0531/0835/b0212.png)
![[CN^-]=0.0000229 M](/tpl/images/0531/0835/20a85.png)
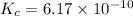
![K_c=\frac{[H^+][CN^-]}{[HCN]}](/tpl/images/0531/0835/77800.png)