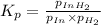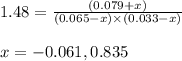Chemistry, 02.03.2020 23:05 shukriabdisabrie
Gaseous indium dihydride is formed from the elements at elevated temperature:
ln(g)+H2(g)?lnH2(g)Kp = 1.48 at 973 K
Partial pressures measured in a reaction vessel are:
PIn = 0.0650atm , PH2 = 0.0330atm , PInH2 = 0.0790atm
1. Calculate Qp
2. Determine the direction of reaction to attain equilibrium
3. Determine the equilibrium partial pressure of In
4. Determine the equilibrium partial pressure of H2
5. Determine the equilibrium partial pressure of InH2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1

Chemistry, 23.06.2019 09:30
What is the best describtion of the side of the moon that faces earth?
Answers: 1
You know the right answer?
Gaseous indium dihydride is formed from the elements at elevated temperature:
ln(g)+H2(g...
ln(g)+H2(g...
Questions

English, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00

Business, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00



Mathematics, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00




Biology, 02.03.2021 06:00


Spanish, 02.03.2021 06:00


Mathematics, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00

Mathematics, 02.03.2021 06:00

English, 02.03.2021 06:00

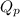 for above reaction is 36.83
for above reaction is 36.83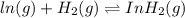
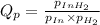
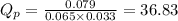
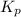 of the reaction = 1.48
of the reaction = 1.48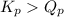 ; the reaction is product favored.When
; the reaction is product favored.When 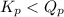 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 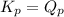 ; the reaction is in equilibrium.
; the reaction is in equilibrium.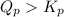 for the given reaction, the reaction is reactant favored.
for the given reaction, the reaction is reactant favored.