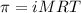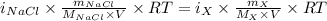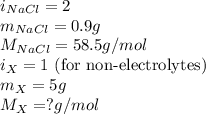Chemistry, 02.03.2020 23:07 bryanmcmillianjr
The osmotic pressure of a 0.9 weight percent solution of sodium chloride (NaCl) is equal to the osmotic pressure of a 5 weight percent solution of non-dissociating molecule X. What is the molecular weight of molecule X

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3


Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
The osmotic pressure of a 0.9 weight percent solution of sodium chloride (NaCl) is equal to the osmo...
Questions




Biology, 29.10.2020 18:40


Mathematics, 29.10.2020 18:40

Health, 29.10.2020 18:40


Mathematics, 29.10.2020 18:40

Mathematics, 29.10.2020 18:40

World Languages, 29.10.2020 18:40

Chemistry, 29.10.2020 18:40



Computers and Technology, 29.10.2020 18:40


History, 29.10.2020 18:40


Mathematics, 29.10.2020 18:40