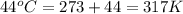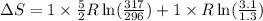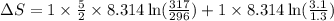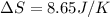Chemistry, 02.03.2020 23:16 camirialchambers17
A sample of helium (He) gas initially at 23°C and 1.0 atm is expanded from 1.3 L to 3.1 L and simultaneously heated to 44°C. Calculate the entropy change for the process.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1


You know the right answer?
A sample of helium (He) gas initially at 23°C and 1.0 atm is expanded from 1.3 L to 3.1 L and simult...
Questions



Mathematics, 24.03.2020 21:26

Mathematics, 24.03.2020 21:26


Law, 24.03.2020 21:26

Mathematics, 24.03.2020 21:26

Mathematics, 24.03.2020 21:26



Mathematics, 24.03.2020 21:26

Mathematics, 24.03.2020 21:26

Mathematics, 24.03.2020 21:26


History, 24.03.2020 21:26

Mathematics, 24.03.2020 21:26

Mathematics, 24.03.2020 21:26

History, 24.03.2020 21:26


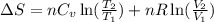
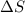 = change in entropy
= change in entropy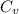 = specific heat capacity at constant volume =
= specific heat capacity at constant volume = 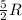 (for monoatomic gas)
(for monoatomic gas)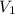 = initial volume of gas = 1.3 L
= initial volume of gas = 1.3 L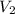 = final volume of gas = 3.1 L
= final volume of gas = 3.1 L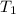 = initial volume of gas =
= initial volume of gas = 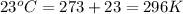
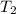 = final volume of gas =
= final volume of gas =