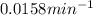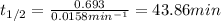Chemistry, 02.03.2020 21:45 michaelandtammytrice
Nuclear decay is a first-order kinetics process. What is the half-life of a radioactive isotope if it takes 233 minutes for the concentration of the isotope to drop from 0.500 M to 0.0125 M? Give your answer in minutes.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1


Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1
You know the right answer?
Nuclear decay is a first-order kinetics process. What is the half-life of a radioactive isotope if i...
Questions

English, 12.03.2020 01:12












Mathematics, 12.03.2020 01:14







![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0530/8019/f1041.png)
![[A_o]](/tpl/images/0530/8019/dc622.png) = initial amount of the reactant = 0.500 M
= initial amount of the reactant = 0.500 M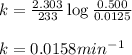
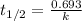
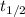 = half-life of the reaction = ?
= half-life of the reaction = ?