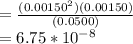Chemistry, 02.03.2020 21:04 oliviacolaizzi
The elementary reaction 2 H 2 O ( g ) − ⇀ ↽ − 2 H 2 ( g ) + O 2 ( g ) proceeds at a certain temperature until the partial pressures of H 2 O , H 2 , and O 2 reach 0.0500 atm, 0.00150 atm, and 0.00150 atm, respectively. What is the value of the equilibrium constant at this temperature?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3
You know the right answer?
The elementary reaction 2 H 2 O ( g ) − ⇀ ↽ − 2 H 2 ( g ) + O 2 ( g ) proceeds at a certain temperat...
Questions


English, 24.06.2021 14:00



Business, 24.06.2021 14:00


English, 24.06.2021 14:00

English, 24.06.2021 14:00

English, 24.06.2021 14:00

Mathematics, 24.06.2021 14:00

English, 24.06.2021 14:00

Mathematics, 24.06.2021 14:00


Mathematics, 24.06.2021 14:00

Biology, 24.06.2021 14:00

Social Studies, 24.06.2021 14:00

English, 24.06.2021 14:00


Biology, 24.06.2021 14:00

Physics, 24.06.2021 14:00

![K_p = \frac{[H_2]^2[O_2]}{H_2O}](/tpl/images/0530/6522/85617.png)