
Chemistry, 02.03.2020 21:07 jahnoibenjamin
Which of the following solutions would have the highest [H3O+]? Assume that they are all 0.10 M in acid at 25∘C. The acid is followed by its Ka value. C) HNO2, 4.6 × 10-4 B) HCN, 4.9 × 10-10 E) HClO2, 1.1 × 10-2 A) HF, 3.5 × 10-4 D) HCHO2, 1.8 × 10-4

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which statement is a reason to support population regulation? a) it is unethical for us to control birth control rates b) humans have the freedom to produce as many children as desired c) the gap between the rich and poor has been narrowing since 1960 d) billions more people on the earth will intensify many environmental and social problems
Answers: 1

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1


Chemistry, 22.06.2019 02:30
At 40 âc the solution has at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.g of kno3 per 100 g of water and it can contain up to at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.g of kno3 per 100 g of water. at 0 âc the solubility is ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.kno3 per 100 g of water, so ~ at 40 â c the solution has blank g of k n o 3 per 100 g of water and it can contain up to blank g of k n o 3 per 100 g of water. at 0 â c the solubility is ~ blank g k n o 3 per 100 g of water, so ~ blank g k n o 3 per 100 g of water will precipitate out of solution.gkno3 per 100 g of water will precipitate out of solution. a kno3 solution containing 55 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 2
You know the right answer?
Which of the following solutions would have the highest [H3O+]? Assume that they are all 0.10 M in a...
Questions

Chemistry, 13.10.2019 12:50



Geography, 13.10.2019 12:50

History, 13.10.2019 12:50




Geography, 13.10.2019 13:00



History, 13.10.2019 13:00




Mathematics, 13.10.2019 13:00


Mathematics, 13.10.2019 13:00


History, 13.10.2019 13:00

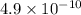
![[H^+]=\sqrt{(K_aC)}](/tpl/images/0530/6657/2da9a.png)
![pH=-\log [H^+]](/tpl/images/0530/6657/37e81.png)
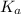 (dissociation constant) increases then the value of hydrogen ion concentration also increases and the value of pH decreases.
(dissociation constant) increases then the value of hydrogen ion concentration also increases and the value of pH decreases.

