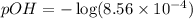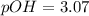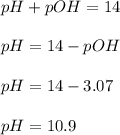Chemistry, 02.03.2020 20:56 eemorales5100
Calculate the pH and pOH of the solutions with the following hydrogen ion or hydroxide ion concentrations. Indicate which solutions are acidic, basic, or neutral. (And please show how to solve! Thanks!) a. [H+]= 3.45x10^-8 M b. [H+]= 2.0x10^-5 M c. [H+]= 7.0x10^-8 M d. [OH-]= 8.56x10^-4 M

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 23.06.2019 03:30
Scientists often deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each of these numbers in an alternate form.
Answers: 3

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2
You know the right answer?
Calculate the pH and pOH of the solutions with the following hydrogen ion or hydroxide ion concentra...
Questions




Mathematics, 18.10.2019 21:00

Geography, 18.10.2019 21:00





Biology, 18.10.2019 21:10

Mathematics, 18.10.2019 21:10

History, 18.10.2019 21:10



Mathematics, 18.10.2019 21:10

Social Studies, 18.10.2019 21:10




![pH=-\log [H^+]](/tpl/images/0530/6113/37e81.png)
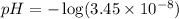
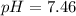
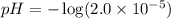
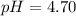
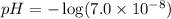
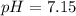
![pOH=-\log [OH^-]](/tpl/images/0530/6113/1fac1.png)