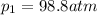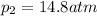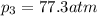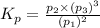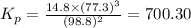Chemistry, 02.03.2020 21:20 gizmo50245
Ammonia decomposes to form nitrogen and hydrogen, like this: Also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, nitrogen, and hydrogen has the following composition: compound pressure at equilibrium. Calculate the value of the equilibrium constant for this reaction.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

You know the right answer?
Ammonia decomposes to form nitrogen and hydrogen, like this: Also, a chemist finds that at a certain...
Questions

History, 26.10.2019 06:43






History, 26.10.2019 06:43

Mathematics, 26.10.2019 06:43


Social Studies, 26.10.2019 06:43


Social Studies, 26.10.2019 06:43


Mathematics, 26.10.2019 06:43

Mathematics, 26.10.2019 06:43

English, 26.10.2019 06:43




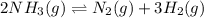
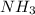 98.8 atm
98.8 atm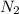 14.8 atm
14.8 atm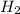 77.3 atm
77.3 atm