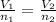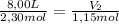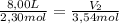Chemistry, 02.03.2020 19:49 barstr9146
A sample containing 2.30 mol of Ne gas has an initial volume of 8.00 L. What is the final volume, in liters, when the following changes occur in the quantity of the gas at constant pressure and temperature?a. A leak allows one-half of Ne atoms to escape. b. A sample of 3.50 mol of Ne is added to the 2.30 mol of Ne gas in the container. c. A sample of 25.0 g of Ne is added to the 2.30 mol of Ne gas in the container.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1


Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
A sample containing 2.30 mol of Ne gas has an initial volume of 8.00 L. What is the final volume, in...
Questions

Mathematics, 07.03.2021 09:20

Mathematics, 07.03.2021 09:20

Mathematics, 07.03.2021 09:20




Mathematics, 07.03.2021 09:20


Physics, 07.03.2021 09:20


History, 07.03.2021 09:20

English, 07.03.2021 09:20


English, 07.03.2021 09:20

Mathematics, 07.03.2021 09:20


English, 07.03.2021 09:20

English, 07.03.2021 09:20

English, 07.03.2021 09:20

Biology, 07.03.2021 09:20