

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
The combustion of propane (C 3H 8) produces CO 2 and H 2O: C 3H 8 (g) 5O2 (g) 3CO2 (g) 4 H 2O (g) Th...
Questions


Mathematics, 18.05.2021 23:30




Spanish, 18.05.2021 23:30


Mathematics, 18.05.2021 23:30


Mathematics, 18.05.2021 23:30










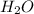 produced will be, 2 moles
produced will be, 2 moles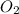 = 2.5 mol
= 2.5 mol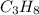 = 4.6 mol
= 4.6 mol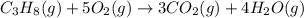
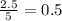 moles of
moles of 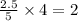 moles of
moles of 

