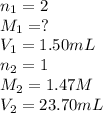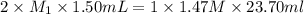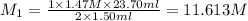Chemistry, 02.03.2020 19:18 coltonsteighner
A 1.50 mL sample of a sulfuric acid solution from an automobile storage battery is titrated with 1.47 M sodium hydroxide solution to a phenolphthalein endpoint, requiring 23.70 mL. What is the molarity of the sulfuric acid solution

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

You know the right answer?
A 1.50 mL sample of a sulfuric acid solution from an automobile storage battery is titrated with 1.4...
Questions


Biology, 13.10.2020 21:01

Mathematics, 13.10.2020 21:01

Mathematics, 13.10.2020 21:01





Mathematics, 13.10.2020 21:01

English, 13.10.2020 21:01




English, 13.10.2020 21:01

Chemistry, 13.10.2020 21:01

Mathematics, 13.10.2020 21:01

Mathematics, 13.10.2020 21:01


Mathematics, 13.10.2020 21:01

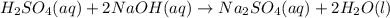
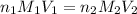
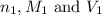 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 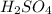
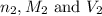 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.