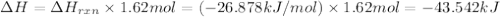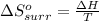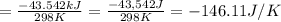Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
I2(g) + Cl2(g)2ICl(g) Using standard thermodynamic data at 298K, calculate the entropy change for th...
Questions



Mathematics, 03.02.2021 17:40

Mathematics, 03.02.2021 17:40

English, 03.02.2021 17:40

Mathematics, 03.02.2021 17:40

History, 03.02.2021 17:40

History, 03.02.2021 17:40

Mathematics, 03.02.2021 17:40





History, 03.02.2021 17:40


History, 03.02.2021 17:40



Mathematics, 03.02.2021 17:40

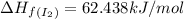
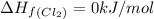
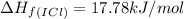
![\Delta H_{rxn}=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0530/3455/db29b.png)
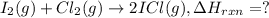
![\Delta H_{rxn}=[(2\times \Delta H_f_{(ICl)})]-[(1\times \Delta H_f_{(I_2)})+(1\times \Delta H_f_{(Cl_2)})]](/tpl/images/0530/3455/4a661.png)
![=[2\times 17.78 kJ/mol]-[1\times 0 kJ/mol+1\times 62.436 kJ/mol]=-26.878 kJ/mol](/tpl/images/0530/3455/6a4a8.png)