Chemistry, 02.03.2020 18:22 hsjsjsjdjjd
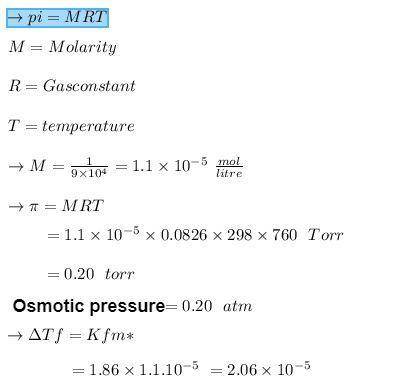
Calculate the freezing-point depression and osmotic pressure at 258C of an aqueous solution containing 1.0 g/L of a protein (molar mass 5 9.0 3 104 g/mol) if the density of the solution is 1.0 g/cm3. b. Considering your answer to part a, which colligative property, freezing-point depression or osmotic pres- sure, would be better used to determine the molar masses of large molecules

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
Calculate the freezing-point depression and osmotic pressure at 258C of an aqueous solution containi...
Questions



Mathematics, 23.01.2020 08:31




Business, 23.01.2020 08:31

Mathematics, 23.01.2020 08:31




Mathematics, 23.01.2020 08:31

History, 23.01.2020 08:31



Mathematics, 23.01.2020 08:31



Mathematics, 23.01.2020 08:31