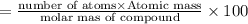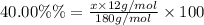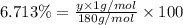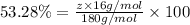Chemistry, 02.03.2020 18:30 leylaanderson85311
A compound consists of 40.00% C, 6.713% H and 53.28% O on a mass basis and has a molar mass of about 180 g/mole. Determine the molecular formula of the compound.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1


Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1
You know the right answer?
A compound consists of 40.00% C, 6.713% H and 53.28% O on a mass basis and has a molar mass of about...
Questions





Biology, 10.03.2020 05:52

Mathematics, 10.03.2020 05:52


Social Studies, 10.03.2020 05:53

History, 10.03.2020 05:53

Mathematics, 10.03.2020 05:53



Mathematics, 10.03.2020 05:53





Mathematics, 10.03.2020 05:54


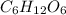 .
.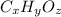 .in
.in