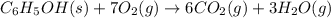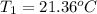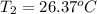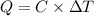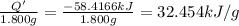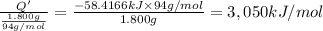A 1.800-g sample of solid phenol (C6H5OH(s)) was burned in a bomb calorimeter whose total heat capacity is 11.66 kJ/?C. The temperature of the calorimeter plus contents increased from 21.36?Cto 26.37?C. Part A
Write a balanced chemical equation for the bomb calorimeter reaction.
Part B:
What is the heat of combustion per gram of phenol?Part C:
Per mole of phenol?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 23.06.2019 10:10
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3

You know the right answer?
A 1.800-g sample of solid phenol (C6H5OH(s)) was burned in a bomb calorimeter whose total heat capac...
Questions



History, 04.02.2020 22:54




English, 04.02.2020 22:54

Social Studies, 04.02.2020 22:55




Social Studies, 04.02.2020 22:55



Mathematics, 04.02.2020 22:55