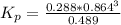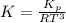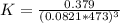Chemistry, 02.03.2020 16:54 cristinanina
Consider the decomposition of the compound C5H6O3 as follows below. C5H6O3(g) → C2H6(g) + 3 CO(g) When a 5.63-g sample of pure C5H6O3(g) was sealed into an otherwise empty 2.50 L flask and heated to 200.°C, the pressure in the flask gradually rose to 1.63 atm and remained at that value. Calculate K for this reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3

Chemistry, 23.06.2019 11:30
Jenny places a strip of ph paper into a solution. when she removes the ph paper, it has turned yellow-green. what should jenny do next to determine the ph of her solution? a. use a different testing method because the ph paper should not change colors b. place the ph paper into a machine that reads the ph of the solution c. compare the ph paper's color with the color of ph paper from another solution d. compare the ph paper's color with a chart of colors and ph ranges
Answers: 1

Chemistry, 23.06.2019 13:30
Which of the following is true regarding chemical and nuclear reactions?
Answers: 1
You know the right answer?
Consider the decomposition of the compound C5H6O3 as follows below. C5H6O3(g) → C2H6(g) + 3 CO(g) Wh...
Questions


Business, 04.05.2020 23:25

Chemistry, 04.05.2020 23:25






Health, 04.05.2020 23:26


Mathematics, 04.05.2020 23:26


History, 04.05.2020 23:26





Chemistry, 04.05.2020 23:26


Mathematics, 04.05.2020 23:26