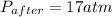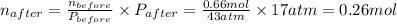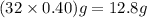A rigid tank contains 0.66 mol of oxygen (O2). Find the mass of oxygen that must be withdrawn from the tank to lower the pressure of the gas from 43 atm to 17 atm. Assume that the volume of the tank and the temperature of the oxygen are constant during this operation. Answer in units of g.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 23.06.2019 00:00
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2
You know the right answer?
A rigid tank contains 0.66 mol of oxygen (O2). Find the mass of oxygen that must be withdrawn from t...
Questions

Arts, 02.11.2020 07:40

Social Studies, 02.11.2020 07:40



Business, 02.11.2020 07:40

Mathematics, 02.11.2020 07:40

Mathematics, 02.11.2020 07:40

History, 02.11.2020 07:40




Mathematics, 02.11.2020 07:40


English, 02.11.2020 07:40




Social Studies, 02.11.2020 07:40


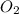 must be withdrawn from tank
must be withdrawn from tank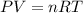
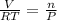
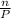 ratio will also be constant before and after removal of
ratio will also be constant before and after removal of 
 and
and