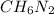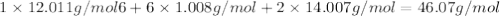Chemistry, 02.03.2020 07:15 snowprincess99447
Determine the molecular formula for the compound with a molar mass of 46.08 g/mol and the following percent compostion:
C:H:N:26.06%13.13%60.81%

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
Determine the molecular formula for the compound with a molar mass of 46.08 g/mol and the following...
Questions

Mathematics, 13.12.2021 04:20

Spanish, 13.12.2021 04:20

Chemistry, 13.12.2021 04:20

Advanced Placement (AP), 13.12.2021 04:20



Advanced Placement (AP), 13.12.2021 04:20

History, 13.12.2021 04:20



Biology, 13.12.2021 04:20


History, 13.12.2021 04:20

Arts, 13.12.2021 04:20

Mathematics, 13.12.2021 04:20

Social Studies, 13.12.2021 04:20

Arts, 13.12.2021 04:20

Mathematics, 13.12.2021 04:20