

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

You know the right answer?
A molecule has the empirical formula C4H6O. If its molecular weight is determined to be about 212 g/...
Questions




English, 01.09.2019 06:30

Mathematics, 01.09.2019 06:30

Mathematics, 01.09.2019 06:30

Social Studies, 01.09.2019 06:30

Mathematics, 01.09.2019 06:30



Mathematics, 01.09.2019 06:30



Mathematics, 01.09.2019 06:30



Arts, 01.09.2019 06:30

Social Studies, 01.09.2019 06:30

History, 01.09.2019 06:30

English, 01.09.2019 06:30

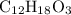 .
. 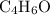 , then the molecular formula would be
, then the molecular formula would be 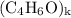 , or equivalently
, or equivalently 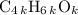 , where
, where 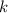 is a positive whole number (
is a positive whole number (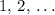 , etc.) The goal here is to find the value of
, etc.) The goal here is to find the value of 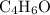 would be
would be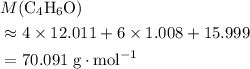 .
.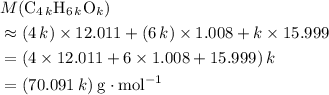 .
.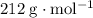 ,
, 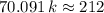 .
.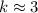 . (Round to the nearest whole number.)
. (Round to the nearest whole number.)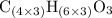 , which simplifies to
, which simplifies to 

