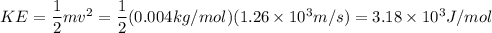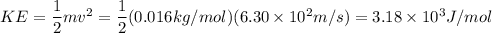Chemistry, 02.03.2020 04:28 jordanwrighta1
At 25.0c helium molecules have an average velocity of 1.26x10^5 cm/s and methane molecukes have an average velocity of 6.30x10^4 cm/s calculate the kinetic energy of each type of molecule at 25.0c and determine which is greater

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1
You know the right answer?
At 25.0c helium molecules have an average velocity of 1.26x10^5 cm/s and methane molecukes have an a...
Questions







Biology, 24.08.2019 13:30



Mathematics, 24.08.2019 13:30



Business, 24.08.2019 13:30

Social Studies, 24.08.2019 13:30


Health, 24.08.2019 13:30