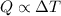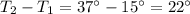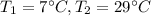Chemistry, 29.02.2020 05:25 kellimcollier8294
A container with a temperature T1 of 15°C is submerged in a bucket of hot water with a temperature T2 of 37°C. An identical container with a temperature of T1 is submerged in an identical bucket of water with a temperature of T2. If the amounts of energy transferred as heat between the containers and the water are the same in both cases, which of the following statements is true?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
You know the right answer?
A container with a temperature T1 of 15°C is submerged in a bucket of hot water with a temperature T...
Questions


Advanced Placement (AP), 20.10.2020 02:01

Mathematics, 20.10.2020 02:01

Physics, 20.10.2020 02:01



Mathematics, 20.10.2020 02:01


Mathematics, 20.10.2020 02:01




Mathematics, 20.10.2020 02:01


English, 20.10.2020 02:01