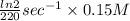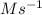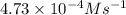Chemistry, 29.02.2020 03:23 tobywaffle1234
A series of experiments investigating the reaction of (CH3)3CCl with H2O to create (CH3)3OH produces a plot of Ln[(CH3)3CCl] vs. time that is linear with a negative slope. Suppose the reaction is carried out under conditions such that the half-life of the reaction is 2.20 x 102 s. What is the instantaneous rate of reaction when [(CH3)3CCl] = 0.15 M?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1


Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2
You know the right answer?
A series of experiments investigating the reaction of (CH3)3CCl with H2O to create (CH3)3OH produces...
Questions


Mathematics, 23.05.2020 23:03


Mathematics, 23.05.2020 23:03

Social Studies, 23.05.2020 23:03


Mathematics, 23.05.2020 23:03

Biology, 23.05.2020 23:03

Computers and Technology, 23.05.2020 23:03

Social Studies, 23.05.2020 23:03


Business, 23.05.2020 23:03

Biology, 23.05.2020 23:03

Mathematics, 23.05.2020 23:03

Mathematics, 23.05.2020 23:03



Biology, 23.05.2020 23:03

Mathematics, 23.05.2020 23:03

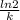
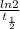
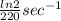
![[(CH_{3})_{3}CCl]^{1}](/tpl/images/0528/9812/8a6e5.png)