

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
You know the right answer?
Calculate the cell potential E at 25°C for the reaction 2 Al(s) + 3 Fe2+(aq) → 2 Al3+(aq) + 3 Fe(s)...
Questions


Mathematics, 14.04.2021 18:20



English, 14.04.2021 18:20

Business, 14.04.2021 18:20

Geography, 14.04.2021 18:20


Mathematics, 14.04.2021 18:20

History, 14.04.2021 18:20

Social Studies, 14.04.2021 18:20



Mathematics, 14.04.2021 18:20

Mathematics, 14.04.2021 18:20

Mathematics, 14.04.2021 18:20

Mathematics, 14.04.2021 18:20

English, 14.04.2021 18:20

Mathematics, 14.04.2021 18:20

Mathematics, 14.04.2021 18:20

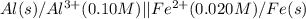
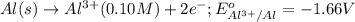
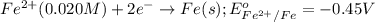
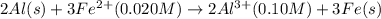
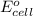 of the reaction, we use the equation:
of the reaction, we use the equation:
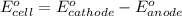
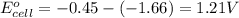
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Al^{3+}]^2}{[Fe^{2+}]^3}](/tpl/images/0528/8715/94ca6.png)
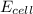 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V



