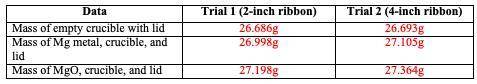
Calculate the theoretical yield of MgO for each trial.
Trial 1:
Trial 2:
De...

Chemistry, 29.02.2020 00:47 cjhenson02
Calculate the theoretical yield of MgO for each trial.
Trial 1:
Trial 2:
Determine the percent yield of MgO for your experiment for each trial.
Trial 1:
Trial 2:
Determine the average percent yield of MgO for the two trials
Please explain how to do this :)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
Questions

Arts, 17.11.2020 19:40

Computers and Technology, 17.11.2020 19:40


Biology, 17.11.2020 19:40


Mathematics, 17.11.2020 19:40




Social Studies, 17.11.2020 19:40







Mathematics, 17.11.2020 19:40



Mathematics, 17.11.2020 19:40



