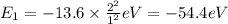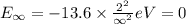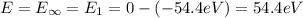Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2
You know the right answer?
Ionization energy is the energy needed to eject an electron from an atom or ion. Calculate the ioniz...
Questions

Biology, 01.07.2019 16:30

Mathematics, 01.07.2019 16:30


Social Studies, 01.07.2019 16:30


History, 01.07.2019 16:30


Mathematics, 01.07.2019 16:30





Mathematics, 01.07.2019 16:30

Biology, 01.07.2019 16:30

Social Studies, 01.07.2019 16:30

Mathematics, 01.07.2019 16:30

English, 01.07.2019 16:30

Mathematics, 01.07.2019 16:30


English, 01.07.2019 16:30

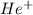 is 54.4 eV.
is 54.4 eV.
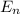 = energy of orbit
= energy of orbit