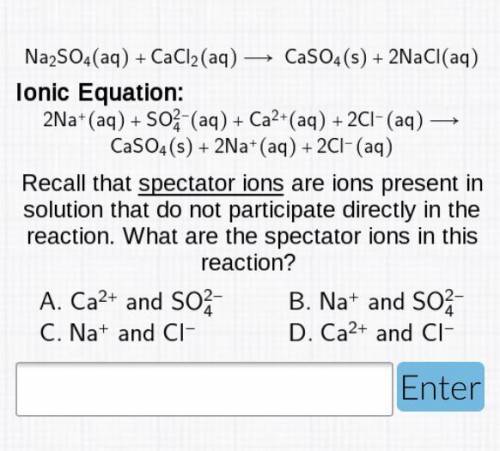
Na2SO4(aq)+CaCl2(aq)—>CaSO4(s)+2 NaCl(aq)
Ionic Equation: 2Na+(aq)+SO2/4-(aq)+Ca2+(aq)+2Cl-...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2


Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
Questions















Computers and Technology, 04.12.2019 06:31

Computers and Technology, 04.12.2019 06:31