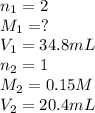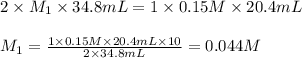Chemistry, 28.02.2020 23:18 smhrosepetals
If it takes 20.4 mL of NaOH(aq) to reach the equivalence point of the titration, what is the molarity of H2SO4(aq)? For your answer, only type in the numerical value with two significant figures. Do NOT include the unit.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

You know the right answer?
If it takes 20.4 mL of NaOH(aq) to reach the equivalence point of the titration, what is the molarit...
Questions

English, 25.09.2019 17:10

Mathematics, 25.09.2019 17:10




Computers and Technology, 25.09.2019 17:10


Mathematics, 25.09.2019 17:10



Mathematics, 25.09.2019 17:10


Biology, 25.09.2019 17:10


Mathematics, 25.09.2019 17:10




Mathematics, 25.09.2019 17:10

Physics, 25.09.2019 17:10

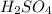 (aq) of an unknown concentration was titrated with 0.15 M of NaOH(aq).
(aq) of an unknown concentration was titrated with 0.15 M of NaOH(aq).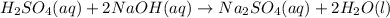
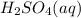 ? For your answer, only type in the numerical value with two significant figures. Do NOT include the unit.
? For your answer, only type in the numerical value with two significant figures. Do NOT include the unit.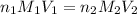
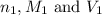 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 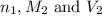 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.