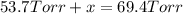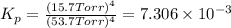Chemistry, 28.02.2020 22:46 AutumnJoy12
In a study of the reaction below at 1181 K, it was observed that when the equilibrium partial pressure of water vapor is 53.7 torr, the total pressure at equilibrium is 69.4 torr. Calculate Kp for this reaction at 1181 K. 3 Fe(s) + 4 H2O(g) equilibrium reaction arrow Fe3O4(s) + 4 H2(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
In a study of the reaction below at 1181 K, it was observed that when the equilibrium partial pressu...
Questions

History, 15.01.2021 06:40


Biology, 15.01.2021 06:40

Advanced Placement (AP), 15.01.2021 06:40

History, 15.01.2021 06:40

Mathematics, 15.01.2021 06:40


Mathematics, 15.01.2021 06:40

Biology, 15.01.2021 06:50


Mathematics, 15.01.2021 06:50

Health, 15.01.2021 06:50



Medicine, 15.01.2021 06:50

Mathematics, 15.01.2021 06:50

Computers and Technology, 15.01.2021 06:50

Mathematics, 15.01.2021 06:50

Mathematics, 15.01.2021 06:50

Health, 15.01.2021 06:50

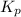 for this reaction at 1181 K is
for this reaction at 1181 K is  .
.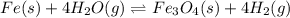
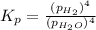
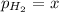
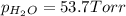
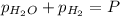 (Dalton's law of partial pressure)
(Dalton's law of partial pressure)