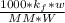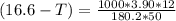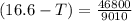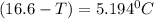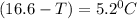Chemistry, 28.02.2020 19:15 noobgirlaskthequest
What is the freezing point of a solution that contains 12.0 g of glucose (C6H12O6) in 50 g of acetic acid (CH3COOH). For acetic acid, Kf is 3.90°C/m and the melting point is 16.6 °C

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 23.06.2019 12:30
What would happen to a weak base dissociation equilibrium if more products we added
Answers: 1
You know the right answer?
What is the freezing point of a solution that contains 12.0 g of glucose (C6H12O6) in 50 g of acetic...
Questions

Mathematics, 17.08.2021 01:00



Computers and Technology, 17.08.2021 01:00


Mathematics, 17.08.2021 01:00


Mathematics, 17.08.2021 01:00

Mathematics, 17.08.2021 01:00


English, 17.08.2021 01:00

Mathematics, 17.08.2021 01:00

Mathematics, 17.08.2021 01:00

Mathematics, 17.08.2021 01:00

Mathematics, 17.08.2021 01:00





Computers and Technology, 17.08.2021 01:10