
Chemistry, 28.02.2020 04:02 TrapQueen665
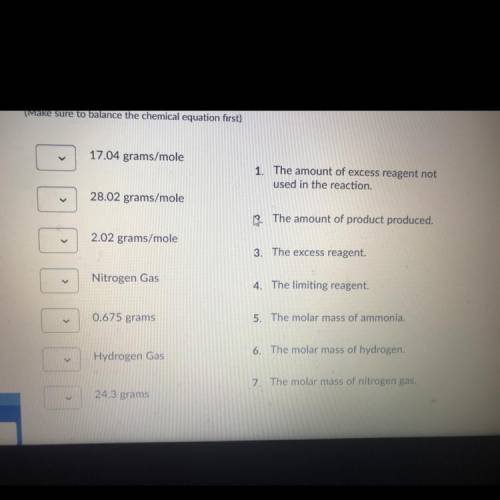
The haber process involves nitrogen gas combining with hydrogen gas to produce ammonia. If 20.0 grams of nitrogen gas combines with 5.0 grams of hydrogen gas, find the following: the molar mass of reactants and products, limiting reactant, the excess reactant, the amount of ammonia produced, the amount of excess chemical not used in the reaction.
Nitrogen gas+ hydrogen gas <——> ammonia gas
N2 + H2 -> NH3
(Make sure to balance the chemical equation first)
A lot of points !!!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
The haber process involves nitrogen gas combining with hydrogen gas to produce ammonia. If 20.0 gram...
Questions


Mathematics, 19.05.2021 01:10

History, 19.05.2021 01:10

Mathematics, 19.05.2021 01:10

Mathematics, 19.05.2021 01:10





Social Studies, 19.05.2021 01:10


Mathematics, 19.05.2021 01:10

Mathematics, 19.05.2021 01:10


Mathematics, 19.05.2021 01:10



Mathematics, 19.05.2021 01:10


English, 19.05.2021 01:10




