
Chemistry, 28.02.2020 02:23 melissapulido198
Consider the equilibrium between SbCl5, SbCl3 and Cl2. SbCl5(g) -->SbCl3(g) + Cl2(g) K = 2.30×10-2 at 566 K .The reaction is allowed to reach equilibrium in a 7.40-L flask. At equilibrium, [SbCl5] = 0.333 M, [SbCl3] = 8.75×10-2 M and [Cl2] = 8.75×10-2 M.
(a) The equilibrium mixture is transferred to a 14.8-L flask. In which direction will the reaction proceed to reach equilibrium?
(b) Calculate the new equilibrium concentrations that result when the equilibrium mixture is transferred to a 14.8-L flask.
[SbCl5] = M
[SbCl3] = M
[Cl2] = M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3

Chemistry, 23.06.2019 04:00
Why must humans find substitutes for many minerals found on earth? (a) form at an extremely slow rate (b) controlled by other countries (c) too deep in the earth to collect
Answers: 1
You know the right answer?
Consider the equilibrium between SbCl5, SbCl3 and Cl2. SbCl5(g) -->SbCl3(g) + Cl2(g) K = 2.30×10-...
Questions

Mathematics, 26.05.2021 20:00

Biology, 26.05.2021 20:00





English, 26.05.2021 20:00

Mathematics, 26.05.2021 20:00

Mathematics, 26.05.2021 20:00

Mathematics, 26.05.2021 20:00

Mathematics, 26.05.2021 20:00



Mathematics, 26.05.2021 20:00


Mathematics, 26.05.2021 20:00

Mathematics, 26.05.2021 20:00


Advanced Placement (AP), 26.05.2021 20:00

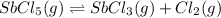
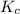
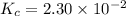
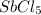 in 7.40 L = 0.333 M
in 7.40 L = 0.333 M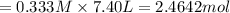
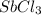 in 7.40 L =
in 7.40 L = 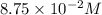
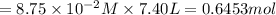
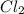 in 7.40 L =
in 7.40 L = 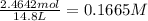
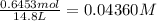
![K_c=\frac{[SbCl_3][Cl_2]}{[SbCl_5]}](/tpl/images/0527/6048/c5c78.png)
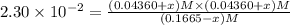
![[SbCl_5]=(0.1665-x) M=(0.1665-0.01536) M=0.1511 M](/tpl/images/0527/6048/35e3f.png)
![[SbCl_3]=(0.04360+x) M=(0.04360+0.01536) M=0.05896 M](/tpl/images/0527/6048/a8c15.png)
![[Cl_2]=(0.04360+x) M=(0.04360+0.01536) M=0.05896 M](/tpl/images/0527/6048/dab14.png)


