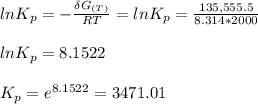Chemistry, 28.02.2020 02:03 Lizzy527663
Calculate the equilibrium composition for the reaction H2−− 1 2O2 −−)−−* H2O when the ratio of the number of moles of elemental hydrogen to elemental oxygen is unity. Perform this calculation at T = 2000 K and P = 1 atm.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 23.06.2019 10:00
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1
You know the right answer?
Calculate the equilibrium composition for the reaction H2−− 1 2O2 −−)−−* H2O when the ratio of the n...
Questions

English, 24.10.2020 03:10

English, 24.10.2020 03:10






Mathematics, 24.10.2020 03:10


Mathematics, 24.10.2020 03:10

Mathematics, 24.10.2020 03:10

Arts, 24.10.2020 03:10



Mathematics, 24.10.2020 03:10

English, 24.10.2020 03:10

Mathematics, 24.10.2020 03:10

Mathematics, 24.10.2020 03:10

Physics, 24.10.2020 03:10

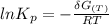 ---------(i)
---------(i)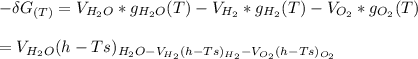
![= V_{H_20[h_f+h_{2000}-hs)-Ts]H_2O - V_{H_2}[h_f+h_{2000}-hs)-Ts]H_2- V_{O_2}[h_f+h_{2000}-hs)-Ts]O_2\\](/tpl/images/0527/5740/e8071.png)