The formation of nitrogen dioxide and oxygen,
NO(g) + O3(g) ⇌ NO2(g) + O2(g)
...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
Questions

Mathematics, 19.09.2019 05:30





Computers and Technology, 19.09.2019 05:30






Social Studies, 19.09.2019 05:30


Mathematics, 19.09.2019 05:30


Mathematics, 19.09.2019 05:30

Mathematics, 19.09.2019 05:30

Mathematics, 19.09.2019 05:30


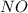 is
is 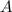 and the initial concentration of
and the initial concentration of 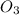 is
is  .
.![R=k[A][B]](/tpl/images/0527/5531/1d87e.png)
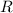 is the rate of reaction. And
is the rate of reaction. And 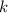 is the rate constant.
is the rate constant.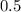 , and the initial concentration of
, and the initial concentration of 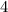 .
.![R'=k[1.5A][4B]\\R'=6k[A][B]\\R'=6R](/tpl/images/0527/5531/f986c.png)



