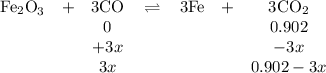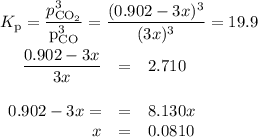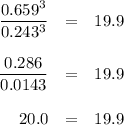Chemistry, 27.02.2020 22:41 bartekpiglo
At 1000 K, Kp=19.9 for the reaction Fe2O3(s)+3CO(g)<--->2Fe(s)+3C O2(g) What are the equilibrium partial pressures of CO and if CO2 is the only gas present initially, at a partial pressure of 0.902 ?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1


Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2
You know the right answer?
At 1000 K, Kp=19.9 for the reaction Fe2O3(s)+3CO(g)<--->2Fe(s)+3C O2(g) What are the equilibri...
Questions

Chemistry, 24.07.2019 06:00


Business, 24.07.2019 06:00



Biology, 24.07.2019 06:00


Mathematics, 24.07.2019 06:00

Biology, 24.07.2019 06:00

History, 24.07.2019 06:00



History, 24.07.2019 06:00



Biology, 24.07.2019 06:00

History, 24.07.2019 06:00


History, 24.07.2019 06:00

History, 24.07.2019 06:10