
Rank the following by their miscibility in
C10H22 (decane), from least miscible to
most: H2O (water), C9H20 (nonane), CH3OH
(methanol), CH3CH2OH (ethanol).
1. CH3OH < CH3CH2OH < C9H20 < H2O
2. H2O < CH3OH < CH3CH2OH < C9H20
3. H2O < CH3CH2OH < CH3OH < C9H20
4. C9H20 < H2O < CH3OH < CH3CH2OH
5. CH3OH < C9H20 < CH3CH2OH < H2O

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3
You know the right answer?
Rank the following by their miscibility in
C10H22 (decane), from least miscible to
most:...
C10H22 (decane), from least miscible to
most:...
Questions

Mathematics, 14.10.2020 16:01

Arts, 14.10.2020 16:01

Biology, 14.10.2020 16:01



Physics, 14.10.2020 16:01

Social Studies, 14.10.2020 16:01


Mathematics, 14.10.2020 16:01

Social Studies, 14.10.2020 16:01



Computers and Technology, 14.10.2020 16:01

Mathematics, 14.10.2020 16:01


Mathematics, 14.10.2020 16:01

English, 14.10.2020 16:01

Mathematics, 14.10.2020 16:01


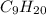 is a non-polar molecule so it is most soluble in decane molecule. Methanol and ethanol are the derivatives of hydrocarbon so they have more solubility than water but less solubility than nonane molecules.
is a non-polar molecule so it is most soluble in decane molecule. Methanol and ethanol are the derivatives of hydrocarbon so they have more solubility than water but less solubility than nonane molecules.



