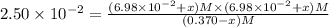Chemistry, 27.02.2020 20:13 SKYBLUE1015
Some SbCl5 is allowed to dissociate into SbCl3 and Cl2 at 521 K. At equilibrium, [SbCl5] = 0.195 M, and [SbCl3] = [Cl2] = 6.98×10-2 M. Additional SbCl5 is added so that [SbCl5]new = 0.370 M and the system is allowed to once again reach equilibrium.
SbCl5(g) <-> SbCl3(g) + Cl2(g) K = 2.50×10-2 at 521 K
(a) In which direction will the reaction proceed to reach equilibrium? to the right to the left
(b) What are the new concentrations of reactants and products after the system reaches equilibrium?
[SbCl5] = M
[SbCl3] = ___M
[Cl2] = ___M

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 23.06.2019 14:00
Ahas distinct properties and composition that never vary.
Answers: 1

Chemistry, 23.06.2019 14:00
Which is not true regarding reaction rates? (2 points) catalysts are not used up in the reaction. catalysts speed up reactions by lowering the activation energy. reaction rates decrease as the concentration of reactants decrease. during reactions, concentrations of all reactants decrease at the same rate.
Answers: 1
You know the right answer?
Some SbCl5 is allowed to dissociate into SbCl3 and Cl2 at 521 K. At equilibrium, [SbCl5] = 0.195 M,...
Questions


Mathematics, 10.12.2021 19:30

English, 10.12.2021 19:30

Social Studies, 10.12.2021 19:30

Mathematics, 10.12.2021 19:30


Mathematics, 10.12.2021 19:30

English, 10.12.2021 19:30


Mathematics, 10.12.2021 19:30

Computers and Technology, 10.12.2021 19:30


Chemistry, 10.12.2021 19:30




Computers and Technology, 10.12.2021 19:30



Mathematics, 10.12.2021 19:30

![[SbCl_5]=(0.370-x) M=(0.370-0.0233) M=0.3467 M](/tpl/images/0527/0501/9b64f.png)
![[SbCl_3]=(6.98\times 10^{-2}+x) M=(6.98\times 10^{-2}+0.0233) M=0.0931 M](/tpl/images/0527/0501/4f238.png)
![[Cl_2]=(6.98\times 10^{-2}+x) M=(6.98\times 10^{-2}+0.0233) M=0.0931 M](/tpl/images/0527/0501/5868d.png)
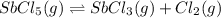
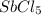 is increasing .So, the equilibrium will shift in the right direction.
is increasing .So, the equilibrium will shift in the right direction.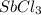 =
= 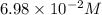
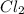 =
= 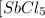 to 0.370 M at equilibrium :
to 0.370 M at equilibrium :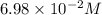
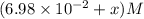
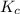
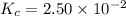
![K_c=\frac{[SbCl_3][Cl_2]}{[SbCl_5]}](/tpl/images/0527/0501/c5c78.png)