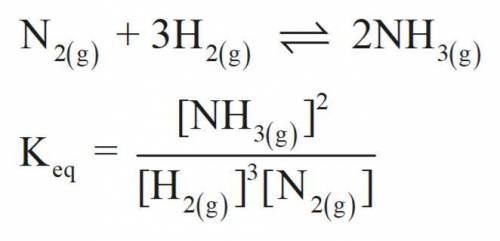Chemistry, 27.02.2020 19:26 Batzs3rdacct
Consider the following chemical equilibrium:
N2(g) + 3H2(g) ⇌ 2NH3
Now write an equation below that shows how to calculate from for this reaction at an absolute temperature . You can assume is comfortably above room temperature.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1

Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1
You know the right answer?
Consider the following chemical equilibrium:
N2(g) + 3H2(g) ⇌ 2NH3
Now writ...
N2(g) + 3H2(g) ⇌ 2NH3
Now writ...
Questions

Mathematics, 17.10.2020 14:01

Social Studies, 17.10.2020 14:01

English, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01


Geography, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01

English, 17.10.2020 14:01




Mathematics, 17.10.2020 14:01