
Be sure to answer all parts. Consider the following equilibrium process at 686°C: CO2(g) + H2(g) ⇌ CO(g) + H2O(g) The equilibrium concentrations of the reacting species are [CO] = 0.0500 M, [H2] = 0.0410 M, [CO2] = 0.0880 M, and [H2O] = 0.0380 M. (a) Calculate Kc for the reaction at 686°C.(b) If we add CO2 to increase its concentrationto 0.30 mol/L, what will theconcentrations of all the gases be when equilibrium isreestablished?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
Be sure to answer all parts. Consider the following equilibrium process at 686°C: CO2(g) + H2(g) ⇌ C...
Questions

Geography, 14.12.2020 22:40

Computers and Technology, 14.12.2020 22:40

Mathematics, 14.12.2020 22:40

History, 14.12.2020 22:40




Geography, 14.12.2020 22:40








Mathematics, 14.12.2020 22:40




Mathematics, 14.12.2020 22:40

![[CO_2]=0.2834 M](/tpl/images/0526/9800/c4d54.png)
![[H_2]=0.02440 M](/tpl/images/0526/9800/4ccc4.png)
![[CO]=0.06660 M](/tpl/images/0526/9800/7fe11.png)
![[H_2O]=0.05460 M](/tpl/images/0526/9800/56dcb.png)
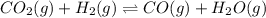
![[CO]=0.0500 M](/tpl/images/0526/9800/16f57.png)
![[H_2]=0.0410 M](/tpl/images/0526/9800/8d6da.png)
![[CO_2]=0.0880 M](/tpl/images/0526/9800/fdfdd.png)
![[H_2O]=0.0380 M](/tpl/images/0526/9800/4cb1c.png)
![K_c=\frac{[CO][H_2O]}{[CO_2][H_2]}](/tpl/images/0526/9800/c597d.png)
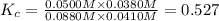
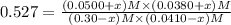
![[CO_2]=(0.30-x)=(0.30-0.0166) M=0.2834 M](/tpl/images/0526/9800/94c3e.png)
![[H_2]=(0.0410-x)=(0.0410-0.0166) M=0.02440 M](/tpl/images/0526/9800/e30dc.png)
![[CO]=(0.0500+x)=(0.0500+0.0166) M=0.06660 M](/tpl/images/0526/9800/af1c7.png)
![[H_2O]=(0.0380+x)=(0.0380+0.0166) M=0.05460 M](/tpl/images/0526/9800/b2417.png)


