Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
You know the right answer?
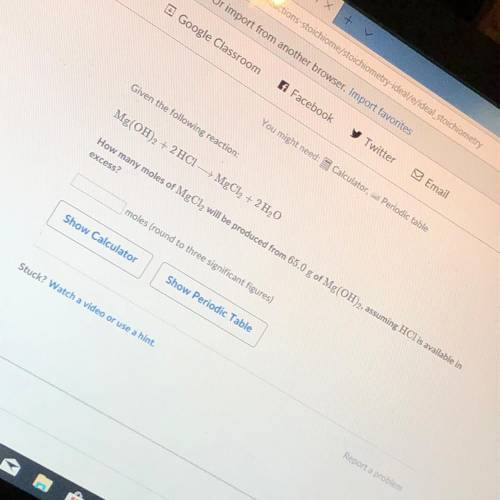
How many moles of MgCl2 will be produced from 65.0 g of Mg(OH)2 assuming HCl is available in excess?...
Questions





Mathematics, 14.03.2020 04:23

Mathematics, 14.03.2020 04:23

Mathematics, 14.03.2020 04:23

Mathematics, 14.03.2020 04:23

English, 14.03.2020 04:23










Computers and Technology, 14.03.2020 04:23