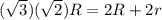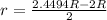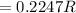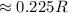Chemistry, 27.02.2020 05:57 linreaburg
(a) Compute the radius r of an impurity atom that will just fit into an FCC octahedral site in terms of the atomic radius R of the host atom (without introducing lattice strains). . (b) Repeat part (a) for the FCC tetrahedral site.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1
You know the right answer?
(a) Compute the radius r of an impurity atom that will just fit into an FCC octahedral site in terms...
Questions

Advanced Placement (AP), 07.02.2021 08:30


English, 07.02.2021 08:30

World Languages, 07.02.2021 08:30



Mathematics, 07.02.2021 08:30

Chemistry, 07.02.2021 08:30

Arts, 07.02.2021 08:30

English, 07.02.2021 08:30







Mathematics, 07.02.2021 08:30

Mathematics, 07.02.2021 08:30

Mathematics, 07.02.2021 08:30

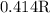
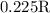 .
.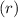 is as shown in the first uploaded image.
is as shown in the first uploaded image.
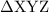 as follows:
as follows: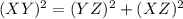
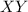 as
as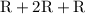 and
and 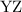 as a and
as a and 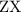 as a in above equation as follows:
as a in above equation as follows: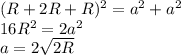
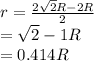
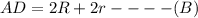
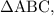
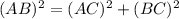
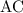 as a and
as a and 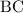 as a in above equation as follows:
as a in above equation as follows: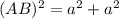
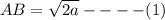
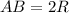
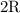 for
for 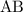 in equation (1) as follows:
in equation (1) as follows: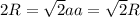
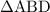 as follows:
as follows: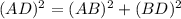
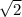 a and
a and  as a in above equation as follows:
as a in above equation as follows: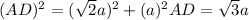
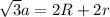
 in above equation as follows:
in above equation as follows: